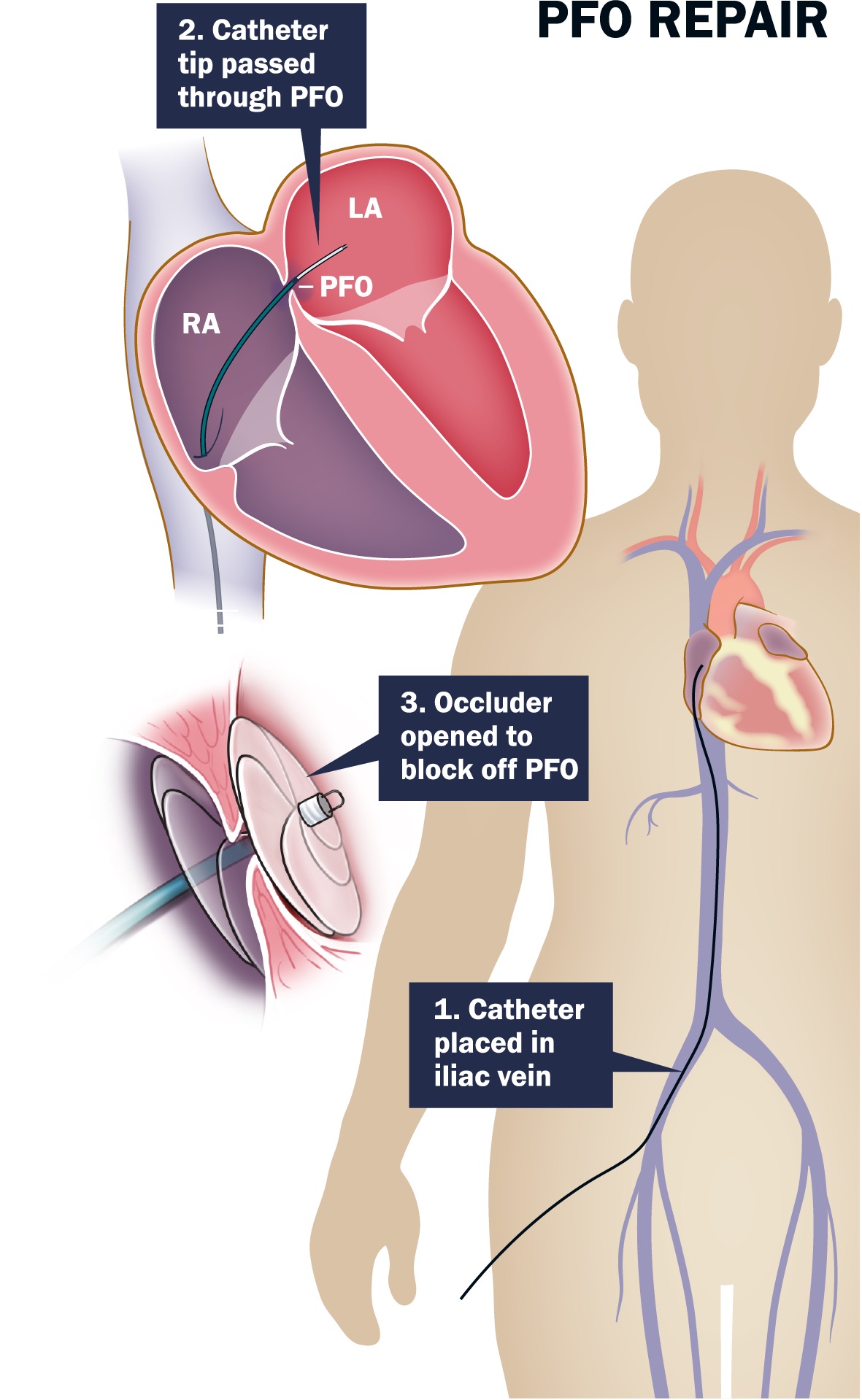
As you may know, patent foramen ovale (PFO) is an opening in the septum or wall between the chambers of the heart, which may not completely close after birth. PFO is present in nearly 25 percent of Americans, yet because it usually causes no symptoms, many are unaware they have PFO. Until, that is, they have a stroke.
For young patients who experience a cryptogenic stroke, PFO may often be the underlying cause. Unfortunately, this puts the patient at risk of recurrent stroke unless addressed.
Preventing recurrent ischemic stroke in this patient population has been a focus of a series of studies at UVA Health System since the late 1990s, with a multidisciplinary team of neurologists and cardiologists actively involved in testing multiple devices designed to occlude the PFO. One of these devices — the AMPLATZER™ PFO Occluder — was recently approved by the U.S. Food and Drug Administration (FDA) and is now available at UVA.
“Although this is a procedure just recently approved by the FDA, we’ve been involved in studying these devices for more than a decade, so we have more experience than anyone else in the state,” says interventional cardiologist Scott Lim, MD, co-director of the UVA Adult Congenital Heart Disease Center.
Watch the video below to learn more about this minimally invasive procedure.
When to Refer
Young patients who experience a stroke should first be evaluated by a neurologist to determine a cause. “After excluding all other causes, then it becomes more likely that PFO is the culprit and the patient should be referred to a cardiologist,” says Lim. “Through a comprehensive evaluation of the underlying issues that may have led to the stroke we can determine how to maintain a patient’s quality of life and choose the best means possible to prevent a second stroke.”
To refer a patient to UVA Heart and Vascular Center, call UVA Physician Direct at 800.522.3723.